Hillsdale Spine Center provides advanced neurosurgical care with Dr. Amritraj Loganathan and Chezlynn Chinavare, Family Nurse Practitioner. The Hillsdale office is located in the orthopedic clinic at 61 W Carleton Road, Hillsdale, Michigan 49242, and in Jackson at 1400 E Michigan Avenue, Jackson, Michigan 49202. All neurosurgeries are performed at Hillsdale Hospital.
Services available at the Hillsdale Spine Center include:
- Cervical and lumbar artificial disc replacement
- Minimally invasive spine surgery
- Spinal fusion
- Discectomy/microdiscectomy
- Laminectomy/laminotomy (decompression surgery)
- Spine fracture stabilization/kyphoplasty
- Spinal cord stimulation
- Robotic-assested spine surgery
- Revision spine surgery
Patients can now make appointments at the Hillsdale location Monday-Friday, 8 am-4:30 pm. To make an appointment at the Jackson location, please call Hillsdale Spine Center and request information about the available dates.
Get in touch with Hillsdale Spine Center by calling (517) 439-5411 and request your preferred location.
Dr. Loganathan is considered a Michigan leader in motion preservation for successful implantations of the prodisc® C Vivo Cervical Total Disc Replacement (TDR) device. The product was approved in July by the by the U.S. Food and Drug Administration (FDA) for 1-level indications and is part of a portfolio of four FDA approved prodisc® C products that allow surgeons to select the implant that best matches a patient’s anatomy and level of disease. Traditional surgical intervention for the spine may include fusing spinal segments together, which permanently fuses two vertebral bodies, eliminating movement between them. A Total Disc Replacement (TDR) offers a surgical alternative to spinal fusions, where the damaged intervertebral disc is removed and replaced with an artificial implant that supports mobility. This technology works to relieve pain in patients suffering from a degenerated spinal disc while enabling motion over the long term at the diseased spinal segment and reducing adjacent-level degeneration and re-operations.
With Centinel Spine, the only United States provider of both Cervical and Lumbar Total Disc Replacement devices, and their prodisc® line of implants (prodisc® C, prodisc® C Vivo, prodisc® C SK, and prodisc® L), Dr. Loganathan ensures the best possible outcomes for his patients.
Spinal cord stimulator (SCS) is a safe, drug-free solution for chronic back pain that hasn’t responded to other therapies. As an FDA-approved pain management therapy, SCS has been clinically proven to help reduce chronic pain by blocking pain signals with a stimulator device connected to nerves in the patient’s back with leads. Dr. Loganathan uses technology from Boston Scientific to provide this treatment.
If you’re interested in SCS therapy, you can try it out with a temporary trial system to see if it works for you. During the trial period, you’ll wear an external stimulator around your waist and experience several days with the therapy before deciding on next steps.
Spinal cord stimulator treatment can be programmed to deliver multiple therapy options. Most programming options offer one of the following techniques.
- Replacing pain signals with a soothing, tingling sensation
- Interrupts pain signals without a tingling sensation
Boston Scientific’s SCS device is unique because it can offer more than one therapy simultaneously. This device is clinically proven to provide greater relief from pain.
One SCS therapy option is Fast™ Therapy, designed for immediate rapid and profound relief. This therapy option can be used during everyday activities like driving or sleeping.
A recent clinical study found that patients using Boston Scientific’s SCS therapies reported a greater improvement in their ability to do everyday activities, compared to non-Boston Scientific SCS options.
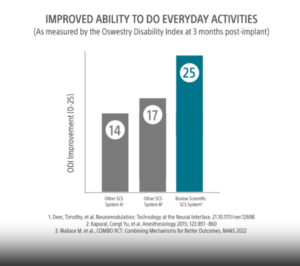
If you opt for this kind of therapy, the device allows for full-body MRIs when certain conditions are met. For more information about the benefits of SCS therapies, talk to a Boston Scientific patient education specialist.


